Circular dichroism in K-shell ionization of fixed in space CO and N2 molecules
|
We have measured the angular distributions of 1s-photoelectrons
excited by circularly and
linearly polarized light from fixed-in-space CO and N2 molecules, in the vicinity of
their shape resonances. A strong circular dichroism, i.e. a strong dependence on the sense of rotation of the polarization
vector of the photons, is found for both molecules. 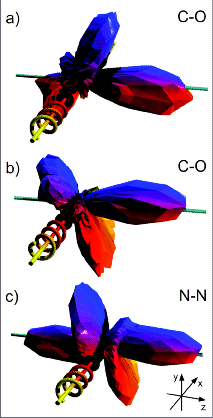 Each vertex of the three-dimensional shape represents one data point. The data have not been smoothed, with the maximum corresponding to about 1000 counts. (c) Analogous distribution of N(1s) photoelectrons (9 eV, on resonance) from N2. 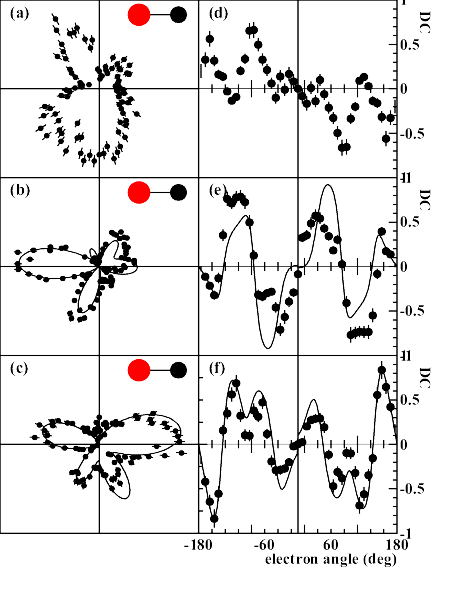 The electron energies are (a)1.6, (b) 10.0 and (c) 24.6 eV. Panels (d-f) show the corresponding circular dichroism. Electron angle 0 corresponds to the direction of the carbon. Full lines: Theoretical multiple scattering calculations for the two higher energies, convoluted with the experimental resolution. |