H2 single ionization - Broken Symmetry
|
The simplest molecule in the
universe, H2, is perfectly symmetric. Its electrons are
delocalized all over the molecule. This is commonly assumed to hold true
while a molecule is dissociating. The well-defined symmetry of the
electron wave function will then create a hole with equal probability at
each of the two fragments.
Lately, we showed
experimental evidence for a third way to break the symmetry of H2:
The electric field of the ejected photoelectron is sufficient to
preferentially localize the bound electron at one side of the remaining
H2+ ion. This retroaction of the photoelectron onto its
parent molecule was recently suggested in pioneering theoretical work by
Serov and Kheifets (V. Serov and A. S. Kheifets, Phys. Rev. A 89, 031402
(2014) but has never been recognized in an experiment.
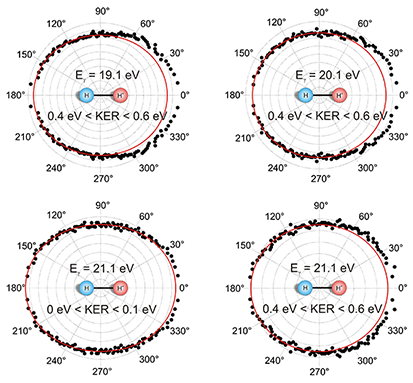
Fig. 1: Angular distribution of the ejected photoelectron. Shown is the angle between the electron momentum vector and the molecular axis for photon energies of 19.1, 20.1, and 21.1 eV. The KER is restricted to intervals from 0 to 0.1 eV and from 0.4 to 0.6 eV, respectively. The red line is a quadratic function of the form a+b(cos(θ))² fitted to the data as a guide for the eye. The molecular orientation is fixed as shown in the middle of the picture. Contrary to these results, ionization and dissociation in two independent steps would lead to symmetric angular distributions. That means that the escaping electron and the fragmentation of the molecule cannot be treated separately and the process can no longer be classified as a Franck-Condon transition. While we have observed this effect in H2, the simplest system where it can occur, we speculate that the effect is general for all symmetric molecules and for all processes ejecting an electron.
In the corresponding publication, our results are discussed in
much more detail and are compared to the theoretical prediction by Serov
and Kheifets:
In a former work, we examined a process where different ionization
pathways involving doubly excited states mix and lead to asymmetric
photo electron angular distributions. Thus the two protons in the
dissociating H2+ molecule can be distinguished.
The experimental data is compared to quantum mechanical ab initio
calculations, which also allows to observe interference effects between
different channels. 
More details can be found here: |